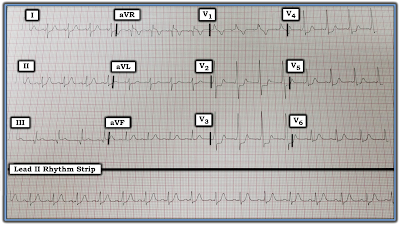
The lead II rhythm strip in Figure-1 was obtained from a middle-aged woman who presented to an out-patient clinic with non-cardiac chest pain.
- What do you see?
- Does this patient have a cardiac problem?
 |
| Figure-1: ECG obtained from a middle-aged woman with non-cardiac chest pain. What do you see? |
=========================
NOTE: The rhythm strip was recorded at the standard speed of 25mm/second. There is slight distortion (slanting) of the tracing. This is unfortunate — because it makes it much more difficult to assess differences in interval duration, which turns out to be very important in this case. That said, the “good news” is that despite this technical shortcoming — we still are able to determine what is going on with this tracing.
- We outline our step-by-step process of analysis below.
=========================
Interpretation: The QRS complex is narrow in this single monitoring lead — so the rhythm appears to be supraventricular. At first glance — the rhythm in Figure-1 looks to be fairly regular. P waves are present — but it looks like the PR interval does not remain constant throughout the tracing.
- PEARL #1: When confronted with a difficult rhythm strip — 2 simple measures greatly facilitate both interpretation, as well as explanation of your approach to others: i) Number the beats in the tracing; and, ii) Label atrial activity with arrows. Numbering the beats saves time and effort — because without this, one is never certain as to which beat is being referred to. Labeling P waves allows you to determine IF there is an underlying sinus rhythm (RED arrows in Figure-2). I have been amazed at how often (and how quickly) simply labeling sinus P waves makes underlying relationships obvious.
- NOTE: Please do not mark up the original rhythm strip with arrows or other notations. The rhythm strip is an official medical record — and if your initial theory turns out to be wrong — then you can’t erase ink markings … So best to make a COPY of the tracing — and then feel free to write on that copy as much as desired.
 |
| Figure-2: We have numbered the beats and labeled P waves (RED arrows). How would you describe the relationship between P waves and QRS complexes in this rhythm strip? |
QUESTION: Take a good look at Figure-2. A fairly (but not completely) regular atrial rhythm is evident (RED arrows).
- How would you describe the relationship between P waves and QRS complexes in Figure-2?
ANSWER: As noted above — there appears to be a fairly regular supraventricular (ie, narrow QRS) rhythm in Figure-2:
- PEARL #2: When dealing with a complex rhythm — Start with what you KNOW! We know the PR interval preceding beat #3 is too short to conduct. The PR interval preceding beats #2 and #6 also appears to be too short to conduct.
- Next — Ask yourself IF there is an underlying sinus rhythm? Note that the PR interval preceding all other beats on this tracing (ie, the PR interval before beats #1; 4,5; 7,8,9) is longer than the PR interval preceding beats #2,3,6 (Figure-3). As alluded to earlier — P waves in this tracing are slightly irregular. But because the PR interval is equal and normal (~0.14 second) preceding beats #1; 4,5; and 7,8,9 — and since P waves are all upright in this lead II monitoring strip — the underlying rhythm is sinus arrhythmia. Since the overall heart rate is under 60/minute — there is also bradycardia.
- Since the 3 beats that are preceded by a PR interval too short to conduct all have a narrow QRS and look similar in morphology to the 6 sinus-conducted beats on this tracing — these 3 beats (ie, beats #2,3,6) must be junctional escape beats.
QUESTION: Look at Figure-3.
- Is there AV dissociation?
- If so — Is there AV block?
ANSWER: By definition — there is transient AV Dissociation in Figure-3. That’s because, at least for some brief period of time — there are some P waves that are not related to neighboring QRS complexes!
- PEARL #3: Just because there is transient AV dissociation does not necessarily mean there is AV block! It is important to realize that there are 3 potential Causes of AV Dissociation: i) AV dissociation due to some form of 2nd or 3rd degree AV Block; ii) AV dissociation by “Usurpation” — in which P waves transiently do not conduct because an accelerated junctional rhythm takes over the pacemaking function (because it is faster than the underlying sinus rhythm); and/or, iii) AV dissociation by “Default” — in which a junctional escape rhythm takes over by “default” (ie, because of SA node slowing).
- PEARL #4: The term, “AV Dissociation” — should never be used as a “diagnosis” per se. Instead — optimal rhythm diagnosis indicates there is AV dissociation because of whichever one or two of the 3 potential causes of this phenomenon is (are) operative. For example, there may be “AV dissociation by usurpation” with Digitalis toxicity — because an overly high dose of Digoxin commonly results in an accelerated junctional rhythm, that then “usurps” control of the underlying sinus rhythm (that is beating at a slower rate).
- PEARL #5: There is no evidence of AV block in Figure-3. In order for there to be 2nd or 3rd-degree AV block — at least some atrial impulses must fail to conduct to the ventricles despite having adequate opportunity for conduction to occur. This never happens in Figure-3. That’s because the P waves preceding beats #2, 3 and 6 never have a chance to conduct, since the PR interval preceding these beats is clearly too short to allow conduction. The only way to determine if some form of AV block might be present in this patient — would be to see a much longer period of monitoring. One needs to see P waves occurring at all points in the cycle at a slow enough rate (usually under 50-60/minute) — before one can judge if some of these P waves are not being conducted despite having adequate opportunity to do so.
QUESTION: Take another look at Figure-3.
- Why is there AV dissociation?
- What are the clinical implications of this finding?
- And — What is the rhythm in Figure-3?
ANSWER: To address these final questions — we measure all R-R intervals on the tracing (Figure-4).
- PEARL #6: You cannot accurately interpret complex arrhythmias unless you regularly use Calipers. IF you have not yet incorporated regular use of calipers into your practice — You will be amazed at how doing so instantly makes you not only “smarter” — but much faster in your assessment of complex arrhythmias.
 |
| Figure-4: Using calipers — we have measured all R-R intervals on this tracing (See text). |
PEARL #7: When confronted with a rhythm strip in which there is transient AV dissociation, and you are not certain as to which beat(s) may be conducting — Look for unexpected shortening of the R-R interval. Such shortening usually indicates which beat(s) is being conducted.
PEARL #8: The above measurements tell us that what we are seeing in Figure 4 is AV Dissociation by “Default” (ie, whenever the sinus pacemaker slows to a rate below 46/minute — the junctional escape rhythm takes over at the appropriate junctional escape rate = 45/minute).
PEARL #9: The clinical implications of the rhythm in Figure-4 depend on the reason for sinus bradycardia. The cause might be iatrogenic — if for example the patient was taking a medication such as a ß-blocker that may overly slow the sinus rate. If this were the case — all that might be needed could be to stop the drug (or reduce the dose). If this resulted in a slightly faster underlying sinus rate — there might no longer be need for a junctional escape rhythm to take over the pacemaking function.
- Most of the time, such shortening will be obvious. However, it is not at all obvious in Figure-4. That said, we can see from our measurements that all sinus-conducted beats in Figure-4 (ie, all beats preceded by P waves with RED arrows) have a shorter preceding R-R interval (ie, between 6.1-to-6.5 large boxes) than the R-R interval preceding the 3 junctional escape beats.
- The R-R interval preceding all 3 junctional escape beats on this tracing = 6.6 large boxes, which corresponds to a junctional escape rate of about 45/minute (ie, 300 ÷ 6.6). This is appropriately within the 40-60/minute normal range for a junctional rhythm.
- The R-R interval preceding the sinus-conducted beats in Figure-4 is between 6.1-to-6.5 large boxes. This corresponds to a sinus bradycardia and arrhythmia with a rate between 46-49/minute.
- NOTE: The rhythm in Figure-4 is not “AV dissociation”. Instead, the rhythm is sinus bradycardia and arrhythmia. It is because of this sinus bradycardia that AV dissociation occurs. And, since we never see P waves that fail to conduct despite having a chance to conduct — there is no evidence on this tracing of any form of AV block ...
- Another potentially benign cause for the rhythm in Figure-4 could be that the patient is a completely healthy endurance athlete, with a “normal-for-her” rhythm of sinus bradycardia and arrhythmia. An intermittent junctional rhythm under such circumstances may be a normal response in a patient without any pathology.
- On the other hand — the reason for sinus bradycardia and arrhythmia might be SSS ( = Sick Sinus Syndrome) — and, perhaps with a longer period of monitoring, evidence of AV block (or more profound sinus rate slowing) might be seen ...
BOTTOM Line: Clinical correlation and a longer period of monitoring is essential for determining IF the rhythm in Figure-4 is pathologic or not. This rhythm might represent a normal, physiologic response in this middle-aged woman with non-cardiac chest pain. Potentially, no other interventions might be needed for such a rhythm. On the other hand — further evaluation might reveal this rhythm to be pathologic and mandate a pacemaker.
- Optimal interpretation for the rhythm in Figure-4 should be: “Sinus bradycardia and arrhythmia, that results in AV dissociation by default — but without evidence of AV block on this tracing”. Optimal management to determine what (if anything) need be done — will depend on clinical correlation.
-------------------------------------------------------------------------
Acknowledgment: My thanks to Dr. Wai Shein from Kyaukpadaung, Myanmar, for his permission allowing me to use this tracing and clinical case.
NOTE: For more on the Basics of AV Block — Please CLICK HERE. My discussion on AV Dissociation begins at 49:25 in this hour-long ECG video.
- For written material regarding the difference between AV Dissociation vs AV Block — See also ECG Blog #21 and/or Section 20 in our ACLS-2013-ePUb.